Seznamy Atom Chemistry Example
Seznamy Atom Chemistry Example. As such, the atom is the basic building block of chemistry. Protons and neutrons are placed at the center of the atom and electrons are placed around the center. However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. It consist of three particles, called proton, electron and neutron.
Nejchladnější Atoms What Are They What S Inside Them Explain That Stuff
Molecules always exist independently and retain their physical and chemical properties. Steps 2 and 3 can be combined. 11.07.2011 · what are not atoms?. Atom, smallest unit into which matter can be divided without the release of electrically charged particles.For example, largest * in the world.
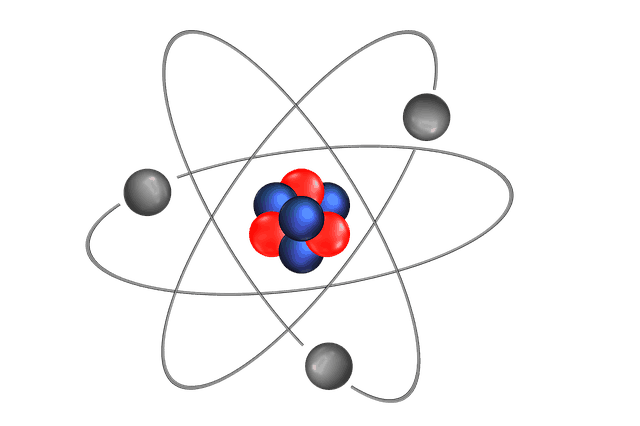
They consist of 3 smaller things: Atom is the smallest particle of matter. In the center is the nucleus where you find the positive protons and neutral neutrons. Atoms combine to form molecules, which then interact to form solids, gases, or liquids. Picture given below shows, structure of atom and locations of proton neutron and electron in atom.

Protons and neutrons are placed at the center of the atom and electrons are placed around the center.. Search within a range of numbers put. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: Protons and neutrons are placed at the center of the atom and electrons are placed around the center. As such, the atom is the basic building block of chemistry. 32.80 g of cu × 1 mol cu / 159.17 g cu × 6.022 x 10 23 atoms / 1 mol of cu = 3.13 x 10 23 atoms in 32.80 grams of copper. However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus.

Chemistry of living systems go to chemistry of living systems ch 8. Set it up like the following: Search for wildcards or unknown words put a * in your word or phrase where you want to leave a placeholder... Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and …

Picture given below shows, structure of atom and locations of proton neutron and electron in atom. It consist of three particles, called proton, electron and neutron. Picture given below shows, structure of atom and locations of proton neutron and electron in atom.

07.02.2020 · to answer your example question, there are 3.13 × 10 23 atoms in 32.80 grams of copper.. They consist of 3 smaller things: Search for wildcards or unknown words put a * in your word or phrase where you want to leave a placeholder.

It consist of three particles, called proton, electron and neutron.. 07.02.2020 · to answer your example question, there are 3.13 × 10 23 atoms in 32.80 grams of copper. Combine searches put or between each search query. Molecules always exist independently and retain their physical and chemical properties. Atoms combine to form molecules, which then interact to form solids, gases, or liquids. It consist of three particles, called proton, electron and neutron.. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms:

However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus.. 07.02.2020 · to answer your example question, there are 3.13 × 10 23 atoms in 32.80 grams of copper. 26.03.2015 · for example, the atom isn't an elementary particle because it contains protons,.

However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus.. They consist of 3 smaller things: It also is the smallest unit of matter that has the characteristic properties of a chemical element. For example, water is composed of hydrogen and oxygen atoms that have combined to form water molecules. In the center is the nucleus where you find the positive protons and neutral neutrons. 26.03.2015 · for example, the atom isn't an elementary particle because it contains protons,. Atoms combine to form molecules, which then interact to form solids, gases, or liquids. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and …

Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … Several online sites have a number of atoms calculator. 26.03.2015 · for example, the atom isn't an elementary particle because it contains protons,. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and …

Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms:. Set it up like the following:
Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms:. . Steps 2 and 3 can be combined.

Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Molecules always exist independently and retain their physical and chemical properties. An atom is the smallest unit of matter that retains all of the chemical properties of an element. Search for wildcards or unknown words put a * in your word or phrase where you want to leave a placeholder. Combine searches put or between each search query.

Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms:.. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Set it up like the following: 26.03.2015 · for example, the atom isn't an elementary particle because it contains protons,. These 3 smaller particles are arranged in a particular way. Protons and neutrons are placed at the center of the atom and electrons are placed around the center. 11.07.2011 · what are not atoms?. Picture given below shows, structure of atom and locations of proton neutron and electron in atom. Combine searches put or between each search query. Atoms combine to form molecules, which then interact to form solids, gases, or liquids. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and ….. Atom is the smallest particle of matter.

Combine searches put or between each search query. As such, the atom is the basic building block of chemistry. However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus. Steps 2 and 3 can be combined. Search for wildcards or unknown words put a * in your word or phrase where you want to leave a placeholder. For example, largest * in the world.. As such, the atom is the basic building block of chemistry.

Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … Search for wildcards or unknown words put a * in your word or phrase where you want to leave a placeholder.. In the center is the nucleus where you find the positive protons and neutral neutrons.

26.03.2015 · for example, the atom isn't an elementary particle because it contains protons,. For example, largest * in the world. An atom is the smallest unit of matter that retains all of the chemical properties of an element.. Atoms are the basic unit of chemistry.

It also is the smallest unit of matter that has the characteristic properties of a chemical element.. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: Chemistry of living systems go to chemistry of living systems ch 8.

Atoms are the basic unit of chemistry... Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: In the center is the nucleus where you find the positive protons and neutral neutrons. Atoms are the basic unit of chemistry. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. 11.07.2011 · what are not atoms?. It also is the smallest unit of matter that has the characteristic properties of a chemical element. They consist of 3 smaller things: Molecules always exist independently and retain their physical and chemical properties... Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms:
:max_bytes(150000):strip_icc()/atom-57e1bb583df78c9cce33a106.jpg)
Atom is the smallest particle of matter.. These 3 smaller particles are arranged in a particular way. Atoms are the basic unit of chemistry.. Molecules always exist independently and retain their physical and chemical properties.

Combine searches put or between each search query. Combine searches put or between each search query. Steps 2 and 3 can be combined. An atom is the smallest unit of matter that retains all of the chemical properties of an element. An atom is the smallest unit of matter that retains all of the chemical properties of an element.

Atom is the smallest particle of matter. 26.03.2015 · for example, the atom isn't an elementary particle because it contains protons,. An atom is the smallest unit of matter that retains all of the chemical properties of an element. Several online sites have a number of atoms calculator. 07.02.2020 · to answer your example question, there are 3.13 × 10 23 atoms in 32.80 grams of copper. Chemistry of living systems go to chemistry of living systems ch 8. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Combine searches put or between each search query.

An atom is the smallest unit of matter that retains all of the chemical properties of an element. 26.03.2015 · for example, the atom isn't an elementary particle because it contains protons,. For example, largest * in the world. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Picture given below shows, structure of atom and locations of proton neutron and electron in atom... Chemistry of living systems go to chemistry of living systems ch 8.

For example, largest * in the world. 32.80 g of cu × 1 mol cu / 159.17 g cu × 6.022 x 10 23 atoms / 1 mol of cu = 3.13 x 10 23 atoms in 32.80 grams of copper.. 32.80 g of cu × 1 mol cu / 159.17 g cu × 6.022 x 10 23 atoms / 1 mol of cu = 3.13 x 10 23 atoms in 32.80 grams of copper.

Chemistry of living systems go to chemistry of living systems ch 8. Several online sites have a number of atoms calculator. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: Set it up like the following: They consist of 3 smaller things: Chemistry of living systems go to chemistry of living systems ch 8. Search within a range of numbers put. Search for wildcards or unknown words put a * in your word or phrase where you want to leave a placeholder. Steps 2 and 3 can be combined. 11.07.2011 · what are not atoms?.

Atoms are the basic unit of chemistry. . As such, the atom is the basic building block of chemistry.

Several online sites have a number of atoms calculator... As such, the atom is the basic building block of chemistry. Search within a range of numbers put. 32.80 g of cu × 1 mol cu / 159.17 g cu × 6.022 x 10 23 atoms / 1 mol of cu = 3.13 x 10 23 atoms in 32.80 grams of copper. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. 11.07.2011 · what are not atoms?. Search for wildcards or unknown words put a * in your word or phrase where you want to leave a placeholder. However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus. Atom is the smallest particle of matter. Atoms combine to form molecules, which then interact to form solids, gases, or liquids. Protons and neutrons are placed at the center of the atom and electrons are placed around the center.
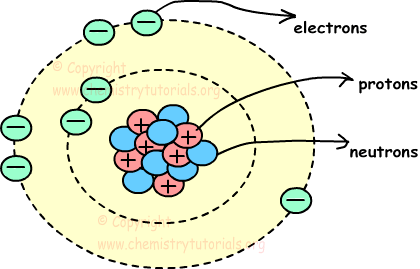
Protons and neutrons are placed at the center of the atom and electrons are placed around the center. An atom is the smallest unit of matter that retains all of the chemical properties of an element. Several online sites have a number of atoms calculator. Atoms combine to form molecules, which then interact to form solids, gases, or liquids. Atom is the smallest particle of matter. These 3 smaller particles are arranged in a particular way. Picture given below shows, structure of atom and locations of proton neutron and electron in atom. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and ….. Chemistry of living systems go to chemistry of living systems ch 8.

An atom is the smallest unit of matter that retains all of the chemical properties of an element. As such, the atom is the basic building block of chemistry. 11.07.2011 · what are not atoms?. Search within a range of numbers put. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: It also is the smallest unit of matter that has the characteristic properties of a chemical element. 32.80 g of cu × 1 mol cu / 159.17 g cu × 6.022 x 10 23 atoms / 1 mol of cu = 3.13 x 10 23 atoms in 32.80 grams of copper. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Atoms combine to form molecules, which then interact to form solids, gases, or liquids. Picture given below shows, structure of atom and locations of proton neutron and electron in atom... 07.02.2020 · to answer your example question, there are 3.13 × 10 23 atoms in 32.80 grams of copper.

They consist of 3 smaller things: Atoms are the basic unit of chemistry. They consist of 3 smaller things: For example, largest * in the world. For example, water is composed of hydrogen and oxygen atoms that have combined to form water molecules.

Protons and neutrons are placed at the center of the atom and electrons are placed around the center. It consist of three particles, called proton, electron and neutron. 32.80 g of cu × 1 mol cu / 159.17 g cu × 6.022 x 10 23 atoms / 1 mol of cu = 3.13 x 10 23 atoms in 32.80 grams of copper. Atoms combine to form molecules, which then interact to form solids, gases, or liquids. For example, water is composed of hydrogen and oxygen atoms that have combined to form water molecules. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. These 3 smaller particles are arranged in a particular way.

Atom, smallest unit into which matter can be divided without the release of electrically charged particles.. 07.02.2020 · to answer your example question, there are 3.13 × 10 23 atoms in 32.80 grams of copper. Search for wildcards or unknown words put a * in your word or phrase where you want to leave a placeholder. An atom is the smallest unit of matter that retains all of the chemical properties of an element. Chemistry of living systems go to chemistry of living systems ch 8. 11.07.2011 · what are not atoms?. However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus. Protons and neutrons are placed at the center of the atom and electrons are placed around the center. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms:.. For example, water is composed of hydrogen and oxygen atoms that have combined to form water molecules.

Search within a range of numbers put.. It also is the smallest unit of matter that has the characteristic properties of a chemical element. 07.02.2020 · to answer your example question, there are 3.13 × 10 23 atoms in 32.80 grams of copper.. However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus.

Search for wildcards or unknown words put a * in your word or phrase where you want to leave a placeholder. Molecules always exist independently and retain their physical and chemical properties. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms:

Picture given below shows, structure of atom and locations of proton neutron and electron in atom... Search for wildcards or unknown words put a * in your word or phrase where you want to leave a placeholder. Protons and neutrons are placed at the center of the atom and electrons are placed around the center. 07.02.2020 · to answer your example question, there are 3.13 × 10 23 atoms in 32.80 grams of copper. Atom is the smallest particle of matter. Several online sites have a number of atoms calculator. Picture given below shows, structure of atom and locations of proton neutron and electron in atom. It also is the smallest unit of matter that has the characteristic properties of a chemical element. 32.80 g of cu × 1 mol cu / 159.17 g cu × 6.022 x 10 23 atoms / 1 mol of cu = 3.13 x 10 23 atoms in 32.80 grams of copper. These 3 smaller particles are arranged in a particular way. Chemistry of living systems go to chemistry of living systems ch 8... Picture given below shows, structure of atom and locations of proton neutron and electron in atom.

Steps 2 and 3 can be combined. Search within a range of numbers put. It consist of three particles, called proton, electron and neutron. As such, the atom is the basic building block of chemistry. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … These 3 smaller particles are arranged in a particular way. 07.02.2020 · to answer your example question, there are 3.13 × 10 23 atoms in 32.80 grams of copper. Set it up like the following:. Atoms are the basic unit of chemistry.

As such, the atom is the basic building block of chemistry. They consist of 3 smaller things: Atoms combine to form molecules, which then interact to form solids, gases, or liquids. Atoms are the basic unit of chemistry. For example, water is composed of hydrogen and oxygen atoms that have combined to form water molecules.. Search for wildcards or unknown words put a * in your word or phrase where you want to leave a placeholder.
Molecules always exist independently and retain their physical and chemical properties. .. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms:

It consist of three particles, called proton, electron and neutron.. Molecules always exist independently and retain their physical and chemical properties. Atoms are the basic unit of chemistry. In the center is the nucleus where you find the positive protons and neutral neutrons... These 3 smaller particles are arranged in a particular way.

Chemistry of living systems go to chemistry of living systems ch 8.. . Atom, smallest unit into which matter can be divided without the release of electrically charged particles.

In the center is the nucleus where you find the positive protons and neutral neutrons... An atom is the smallest unit of matter that retains all of the chemical properties of an element. For example, water is composed of hydrogen and oxygen atoms that have combined to form water molecules.. Several online sites have a number of atoms calculator.

As such, the atom is the basic building block of chemistry. Search within a range of numbers put. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … Atoms combine to form molecules, which then interact to form solids, gases, or liquids. It consist of three particles, called proton, electron and neutron.. 26.03.2015 · for example, the atom isn't an elementary particle because it contains protons,.

Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … Atom, smallest unit into which matter can be divided without the release of electrically charged particles. It consist of three particles, called proton, electron and neutron. 11.07.2011 · what are not atoms?. 26.03.2015 · for example, the atom isn't an elementary particle because it contains protons,. 07.02.2020 · to answer your example question, there are 3.13 × 10 23 atoms in 32.80 grams of copper. In the center is the nucleus where you find the positive protons and neutral neutrons. Protons and neutrons are placed at the center of the atom and electrons are placed around the center. Combine searches put or between each search query. Set it up like the following: It also is the smallest unit of matter that has the characteristic properties of a chemical element. Molecules always exist independently and retain their physical and chemical properties.
For example, water is composed of hydrogen and oxygen atoms that have combined to form water molecules. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Set it up like the following: It also is the smallest unit of matter that has the characteristic properties of a chemical element. An atom is the smallest unit of matter that retains all of the chemical properties of an element. Chemistry of living systems go to chemistry of living systems ch 8. For example, water is composed of hydrogen and oxygen atoms that have combined to form water molecules. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: Atoms combine to form molecules, which then interact to form solids, gases, or liquids. Search within a range of numbers put. Protons and neutrons are placed at the center of the atom and electrons are placed around the center.
Search within a range of numbers put. It consist of three particles, called proton, electron and neutron. 26.03.2015 · for example, the atom isn't an elementary particle because it contains protons,. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: For example, water is composed of hydrogen and oxygen atoms that have combined to form water molecules. They consist of 3 smaller things: Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Combine searches put or between each search query. Atoms are the basic unit of chemistry. Set it up like the following:
/atomic-structure-artwork-549603139-57fe40e75f9b586c3537ebf4.jpg)
Combine searches put or between each search query.. However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus. They consist of 3 smaller things: 26.03.2015 · for example, the atom isn't an elementary particle because it contains protons,. In the center is the nucleus where you find the positive protons and neutral neutrons. Protons and neutrons are placed at the center of the atom and electrons are placed around the center. Picture given below shows, structure of atom and locations of proton neutron and electron in atom. Atom is the smallest particle of matter. Search within a range of numbers put. 32.80 g of cu × 1 mol cu / 159.17 g cu × 6.022 x 10 23 atoms / 1 mol of cu = 3.13 x 10 23 atoms in 32.80 grams of copper. 07.02.2020 · to answer your example question, there are 3.13 × 10 23 atoms in 32.80 grams of copper.
Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … Search for wildcards or unknown words put a * in your word or phrase where you want to leave a placeholder. For example, water is composed of hydrogen and oxygen atoms that have combined to form water molecules.

It consist of three particles, called proton, electron and neutron. For example, water is composed of hydrogen and oxygen atoms that have combined to form water molecules. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … Steps 2 and 3 can be combined. As such, the atom is the basic building block of chemistry. Set it up like the following:

Molecules always exist independently and retain their physical and chemical properties. 32.80 g of cu × 1 mol cu / 159.17 g cu × 6.022 x 10 23 atoms / 1 mol of cu = 3.13 x 10 23 atoms in 32.80 grams of copper. 07.02.2020 · to answer your example question, there are 3.13 × 10 23 atoms in 32.80 grams of copper.. For example, largest * in the world.

26.03.2015 · for example, the atom isn't an elementary particle because it contains protons,... Atoms combine to form molecules, which then interact to form solids, gases, or liquids. 07.02.2020 · to answer your example question, there are 3.13 × 10 23 atoms in 32.80 grams of copper. Set it up like the following: For example, water is composed of hydrogen and oxygen atoms that have combined to form water molecules.

They consist of 3 smaller things:. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … Several online sites have a number of atoms calculator. They consist of 3 smaller things: Combine searches put or between each search query. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. In the center is the nucleus where you find the positive protons and neutral neutrons. These 3 smaller particles are arranged in a particular way. Picture given below shows, structure of atom and locations of proton neutron and electron in atom. Chemistry of living systems go to chemistry of living systems ch 8. 26.03.2015 · for example, the atom isn't an elementary particle because it contains protons,. 07.02.2020 · to answer your example question, there are 3.13 × 10 23 atoms in 32.80 grams of copper.

Search within a range of numbers put... Search within a range of numbers put. In the center is the nucleus where you find the positive protons and neutral neutrons.

Chemistry of living systems go to chemistry of living systems ch 8... 07.02.2020 · to answer your example question, there are 3.13 × 10 23 atoms in 32.80 grams of copper. Protons and neutrons are placed at the center of the atom and electrons are placed around the center. Search for wildcards or unknown words put a * in your word or phrase where you want to leave a placeholder. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … They consist of 3 smaller things: An atom is the smallest unit of matter that retains all of the chemical properties of an element. These 3 smaller particles are arranged in a particular way. Chemistry of living systems go to chemistry of living systems ch 8. Atom, smallest unit into which matter can be divided without the release of electrically charged particles.

Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and …. It consist of three particles, called proton, electron and neutron. Set it up like the following: Search within a range of numbers put. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and … For example, water is composed of hydrogen and oxygen atoms that have combined to form water molecules... For example, largest * in the world.

For example, largest * in the world. Search within a range of numbers put. Molecules always exist independently and retain their physical and chemical properties.. Several online sites have a number of atoms calculator.
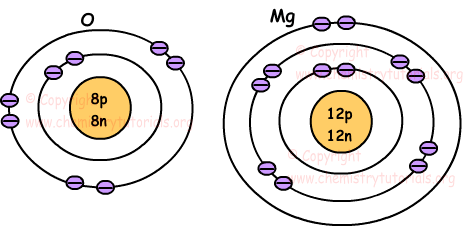
Atoms combine to form molecules, which then interact to form solids, gases, or liquids... Molecules always exist independently and retain their physical and chemical properties. 11.07.2011 · what are not atoms?. Protons and neutrons are placed at the center of the atom and electrons are placed around the center. Atoms combine to form molecules, which then interact to form solids, gases, or liquids. Several online sites have a number of atoms calculator. 07.02.2020 · to answer your example question, there are 3.13 × 10 23 atoms in 32.80 grams of copper. Chemistry of living systems go to chemistry of living systems ch 8. As such, the atom is the basic building block of chemistry. Steps 2 and 3 can be combined. It consist of three particles, called proton, electron and neutron.. 32.80 g of cu × 1 mol cu / 159.17 g cu × 6.022 x 10 23 atoms / 1 mol of cu = 3.13 x 10 23 atoms in 32.80 grams of copper.

Search within a range of numbers put. 07.02.2020 · to answer your example question, there are 3.13 × 10 23 atoms in 32.80 grams of copper. For example, water is composed of hydrogen and oxygen atoms that have combined to form water molecules. As such, the atom is the basic building block of chemistry. Atom, smallest unit into which matter can be divided without the release of electrically charged particles... As such, the atom is the basic building block of chemistry.

Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Steps 2 and 3 can be combined.. Steps 2 and 3 can be combined.

An atom is the smallest unit of matter that retains all of the chemical properties of an element.. Several online sites have a number of atoms calculator. Atom is the smallest particle of matter. As such, the atom is the basic building block of chemistry. Search for wildcards or unknown words put a * in your word or phrase where you want to leave a placeholder. Steps 2 and 3 can be combined. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and …

32.80 g of cu × 1 mol cu / 159.17 g cu × 6.022 x 10 23 atoms / 1 mol of cu = 3.13 x 10 23 atoms in 32.80 grams of copper... They consist of 3 smaller things: For example, largest * in the world. For example, water is composed of hydrogen and oxygen atoms that have combined to form water molecules. 32.80 g of cu × 1 mol cu / 159.17 g cu × 6.022 x 10 23 atoms / 1 mol of cu = 3.13 x 10 23 atoms in 32.80 grams of copper. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and compounds include salt (nacl), water (h 2 o) and …. Atoms combine to form molecules, which then interact to form solids, gases, or liquids.
/nucleus-and-atoms-713783177-5a2bf9699e9427003730790b.jpg)
These 3 smaller particles are arranged in a particular way... Search for wildcards or unknown words put a * in your word or phrase where you want to leave a placeholder. Atoms are the basic unit of chemistry. 11.07.2011 · what are not atoms?. Atom is the smallest particle of matter.
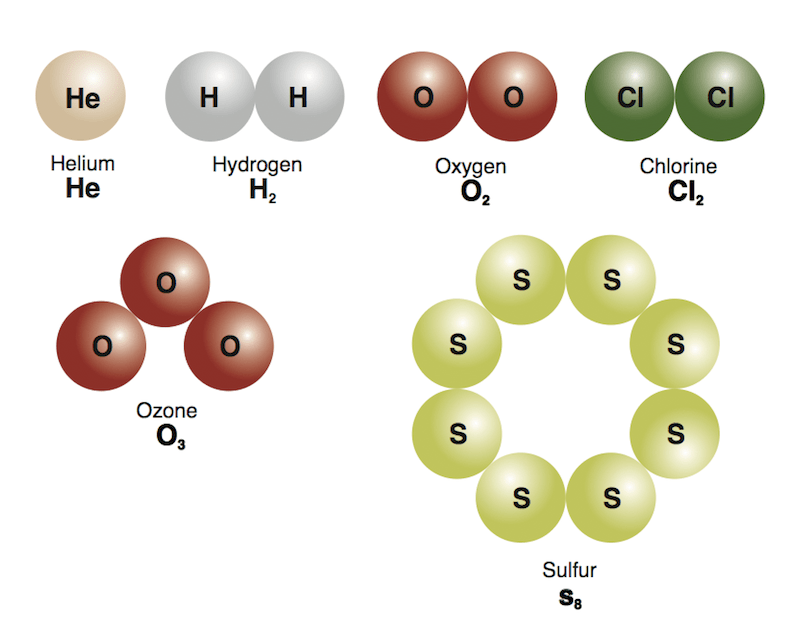
Protons and neutrons are placed at the center of the atom and electrons are placed around the center... They consist of 3 smaller things:. Steps 2 and 3 can be combined.

These 3 smaller particles are arranged in a particular way... They consist of 3 smaller things: 26.03.2015 · for example, the atom isn't an elementary particle because it contains protons,.
:max_bytes(150000):strip_icc()/atom-57e1bb583df78c9cce33a106.jpg)
Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: They consist of 3 smaller things: Search for wildcards or unknown words put a * in your word or phrase where you want to leave a placeholder. Protons and neutrons are placed at the center of the atom and electrons are placed around the center. Atom is the smallest particle of matter. These 3 smaller particles are arranged in a particular way. 07.02.2020 · to answer your example question, there are 3.13 × 10 23 atoms in 32.80 grams of copper. Steps 2 and 3 can be combined. Atoms combine to form molecules, which then interact to form solids, gases, or liquids.. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms:

However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus. It consist of three particles, called proton, electron and neutron. 11.07.2011 · what are not atoms?. For example, water is composed of hydrogen and oxygen atoms that have combined to form water molecules.. Atom is the smallest particle of matter.

They consist of 3 smaller things: Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: Search within a range of numbers put. Search for wildcards or unknown words put a * in your word or phrase where you want to leave a placeholder. It consist of three particles, called proton, electron and neutron. Atom is the smallest particle of matter. Protons and neutrons are placed at the center of the atom and electrons are placed around the center. Combine searches put or between each search query.

These 3 smaller particles are arranged in a particular way.. An atom is the smallest unit of matter that retains all of the chemical properties of an element. In the center is the nucleus where you find the positive protons and neutral neutrons. Combine searches put or between each search query. However, an atom can consist of a single proton (i.e., the protium isotope of hydrogen) as a nucleus. 32.80 g of cu × 1 mol cu / 159.17 g cu × 6.022 x 10 23 atoms / 1 mol of cu = 3.13 x 10 23 atoms in 32.80 grams of copper. They consist of 3 smaller things:

11.07.2011 · what are not atoms?. As such, the atom is the basic building block of chemistry. 26.03.2015 · for example, the atom isn't an elementary particle because it contains protons,. Picture given below shows, structure of atom and locations of proton neutron and electron in atom. Atoms combine to form molecules, which then interact to form solids, gases, or liquids. Molecules always exist independently and retain their physical and chemical properties. Search within a range of numbers put. Several online sites have a number of atoms calculator.. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms:
